How to rebuild T cells

T cells, a critical component of our immune system, play a pivotal role in recognizing and attacking foreign invaders. However, aging, chronic diseases, and certain treatments can deplete or impair T cells, compromising our ability to fight infections and maintain immune balance. By understanding the mechanisms of T cell depletion and investigating novel strategies, researchers are opening up promising avenues to rebuild T cells, offering hope for improved immune function and resilience.
Rebuilding T Cell Function: Strategies and Approaches
Rebuilding or restoring T cell function is a complex process with significant implications for immunotherapy and treating immunodeficiency disorders. It's not about creating entirely new T cells from scratch in the same way you'd grow a new organ (though research in this direction is ongoing with induced pluripotent stem cells). Instead, it focuses on enhancing the function of existing T cells or improving their production and maturation. This involves various approaches targeting different aspects of T cell biology.
1. Enhancing T Cell Proliferation and Expansion
One key strategy for “rebuilding” T cell function is to boost their numbers and activity. This can be achieved through various methods, including: Cytokine therapy, which uses proteins like interleukin-2 (IL-2) or other immunostimulatory cytokines to stimulate T cell growth; Adoptive cell transfer (ACT), where T cells are removed from a patient, expanded in the lab (often genetically modified for enhanced function), and then re-infused; and Immune checkpoint inhibitors, which block proteins that suppress T cell activation, allowing them to proliferate and attack targets more effectively.
2. Improving T Cell Differentiation and Function
T cells aren't a homogeneous population; they differentiate into various subsets with distinct roles (e.g., cytotoxic T cells, helper T cells). To improve T cell function, it's crucial to guide their differentiation toward more effective phenotypes. This often involves manipulating the cytokine milieu during T cell expansion and using strategies to enhance their ability to recognize and kill target cells (e.g., increasing their expression of specific receptors). Furthermore, research is looking at epigenetic modifications to achieve this improved differentiation.
3. Addressing T Cell Exhaustion
Chronic infections and cancers can lead to T cell exhaustion, a state where T cells lose their ability to function effectively. Reversing T cell exhaustion is a major focus of immunotherapy research. Strategies include using checkpoint inhibitors to release the "brakes" on T cell activation, employing cytokine therapy to rejuvenate exhausted T cells, and exploring novel approaches like gene editing to correct the underlying defects associated with exhaustion.
4. Correcting T Cell Developmental Defects
In some immunodeficiencies, T cell function is compromised due to defects in their development. These defects can range from problems with T cell precursor generation to issues with T cell receptor signaling. Approaches to address these developmental defects include gene therapy to correct the underlying genetic mutations and bone marrow transplantation to replace defective hematopoietic stem cells, which give rise to T cells.
5. Utilizing CAR T-cell Therapy
Chimeric antigen receptor (CAR) T-cell therapy represents a powerful approach to rebuild T cell function in the context of cancer treatment. This involves genetically engineering a patient's T cells to express CARs, artificial receptors that recognize and bind specific cancer antigens. The modified T cells are then infused back into the patient, where they effectively target and destroy cancer cells. However, challenges remain including potential toxicity and the development of resistance.
| Strategy | Mechanism | Clinical Applications |
|---|---|---|
| Cytokine Therapy | Stimulates T cell proliferation and activation. | Various cancers, immunodeficiencies. |
| Adoptive Cell Transfer (ACT) | Expansion and re-infusion of patient's own T cells (often modified). | Cancers, viral infections. |
| Immune Checkpoint Inhibitors | Blocks inhibitory signals, enhancing T cell activity. | Cancers. |
| Gene Therapy | Corrects genetic defects in T cell development or function. | Immunodeficiencies, certain cancers. |
| CAR T-cell Therapy | Engineered T cells with specific cancer-targeting receptors. | Specific types of cancers (e.g., leukemia, lymphoma). |
How can I increase my T cells naturally?

How Can I Increase My T Cells Naturally?
Naturally boosting your T cell count requires a holistic approach focusing on lifestyle modifications and dietary choices that support immune function. There's no single magic bullet, and it's crucial to understand that severely compromised immune systems might require medical intervention. The following strategies can contribute to a healthier immune system, which includes a higher T cell count, but it's always best to consult with a healthcare professional before making significant dietary or lifestyle changes, especially if you have pre-existing health conditions.
Optimize Your Diet for Immune Support
Nutrition plays a pivotal role in immune function. A diet rich in fruits, vegetables, and whole grains provides the essential vitamins, minerals, and antioxidants necessary for optimal T cell production and activity. Conversely, processed foods, excessive sugar, and unhealthy fats can negatively impact your immune system. Focusing on nutrient-dense foods is key to supporting your T cell count.
- Increase your intake of Vitamin C, Vitamin D, and Zinc: These are crucial for immune function and T cell development.
- Consume foods rich in antioxidants: Berries, leafy greens, and colorful vegetables are packed with antioxidants that combat oxidative stress, protecting your immune cells.
- Prioritize whole grains, legumes, and lean proteins: These provide the building blocks for cell repair and growth.
Prioritize Sufficient Sleep
Sleep deprivation significantly weakens your immune system, including your T cell response. During sleep, your body repairs and regenerates cells, including T cells. Aim for 7-9 hours of quality sleep each night to support optimal immune function. Establish a regular sleep schedule and create a relaxing bedtime routine to improve sleep quality.
- Maintain a consistent sleep schedule, even on weekends.
- Create a relaxing bedtime routine, such as taking a warm bath or reading a book.
- Ensure your bedroom is dark, quiet, and cool.
Manage Stress Effectively
Chronic stress significantly suppresses the immune system, reducing T cell activity and making you more susceptible to infections. Effective stress management techniques are crucial for maintaining a healthy immune response. Finding healthy ways to cope with stress is vital for supporting your T cell count.
- Practice regular exercise: Physical activity helps reduce stress hormones and boosts immune function.
- Engage in relaxation techniques: Yoga, meditation, or deep breathing exercises can help manage stress.
- Prioritize social connections: Strong social support networks can buffer the negative effects of stress.
Engage in Regular Exercise
Regular physical activity boosts your immune system. Moderate exercise increases blood circulation, improves lymphatic drainage (important for immune cell movement), and reduces inflammation. However, avoid overtraining, as excessive exercise can have the opposite effect and suppress immune function. Finding the right balance is key.
- Aim for at least 30 minutes of moderate-intensity exercise most days of the week.
- Choose activities you enjoy to ensure adherence.
- Listen to your body and rest when needed.
Maintain a Healthy Gut Microbiome
The gut microbiome plays a crucial role in immune regulation, including T cell development and function. A diverse and balanced gut microbiome supports a healthy immune system. Consuming prebiotic and probiotic foods or supplements can contribute to a healthy gut.
- Consume foods rich in prebiotics, such as fruits, vegetables, and whole grains.
- Include probiotic-rich foods like yogurt, kefir, and sauerkraut in your diet.
- Consider a probiotic supplement after consulting with a healthcare professional.
Can you regenerate T cells?
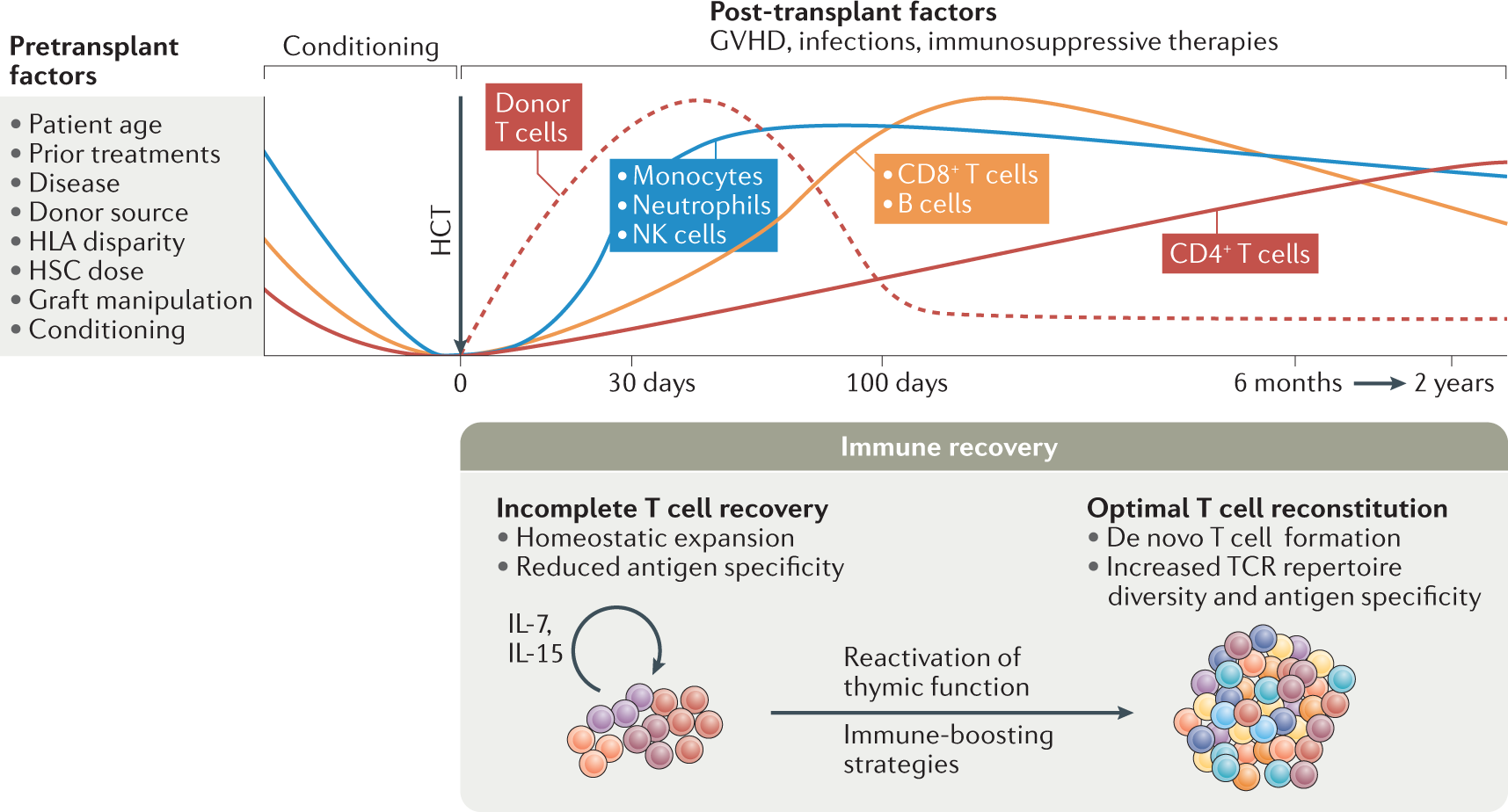
The ability to regenerate T cells is a complex issue, and the answer is nuanced. While the body constantly produces new T cells from hematopoietic stem cells in the bone marrow, the process is not a simple regeneration in the sense of replacing lost cells with identical copies. Instead, it's a continuous process of creation, selection, and differentiation. The extent to which T cell regeneration can effectively compensate for loss or damage depends on various factors, including the age of the individual, the underlying cause of T cell depletion, and the overall health of the immune system. Complete regeneration of a specific, highly specialized T cell population following significant loss is not currently achievable. However, research is actively exploring ways to enhance and manipulate this process to treat immunodeficiency and improve immune responses.
Can the body naturally regenerate T cells?
Yes, the body naturally regenerates T cells throughout life. This process, known as lymphopoiesis, occurs primarily in the bone marrow and thymus. Hematopoietic stem cells differentiate into T cell progenitors, which then migrate to the thymus to mature. This maturation process involves positive and negative selection, ensuring that only T cells that recognize foreign antigens and don't attack self-antigens survive. However, the rate and effectiveness of this natural regeneration decline with age and can be compromised by various diseases or conditions. This natural regeneration is not always sufficient to fully replace substantial T cell loss.
- Bone marrow: The source of hematopoietic stem cells.
- Thymus: The site of T cell maturation and selection.
- Peripheral lymphoid organs: Where mature T cells reside and respond to antigens.
What factors influence T cell regeneration?
Several factors influence the body's ability to regenerate T cells. Age is a significant factor, as the thymus shrinks with age, reducing the production of new T cells. Underlying health conditions, such as HIV infection, autoimmune diseases, or cancer, can severely impair T cell regeneration. Nutritional deficiencies can also affect the process, as essential nutrients are necessary for cell growth and differentiation. Genetic factors may influence the efficiency of T cell regeneration. Furthermore, the type and extent of T cell loss also plays a crucial role. For example, a gradual loss might be compensated for more easily than a sudden, massive depletion.
- Age-related thymic involution
- Disease-induced immune suppression
- Nutritional deficiencies affecting hematopoiesis
Can T cell regeneration be enhanced?
Research is actively exploring ways to enhance T cell regeneration, particularly in contexts of immunodeficiency or after significant T cell loss. Strategies include thymic rejuvenation, where efforts are made to restore the function of the aging thymus. This may involve using drugs or growth factors to stimulate thymic growth and activity. Another approach focuses on manipulating the bone marrow to increase the production of T cell precursors. Adoptive T cell transfer, a process where mature T cells are grown in the lab and then transferred back to the patient, is also being developed to boost immune function. The effectiveness of these approaches varies significantly, and much research is still required.
- Thymic rejuvenation therapies
- Bone marrow stimulation to enhance T cell production
- Adoptive T cell transfer therapies
What are the limitations of T cell regeneration?
While the body constantly generates new T cells, there are limitations to this process. The thymus's capacity to produce new T cells declines significantly with age, leading to an overall decline in immune function. Severe immune damage, such as that caused by HIV or chemotherapy, can overwhelm the body's ability to regenerate T cells effectively. Furthermore, regenerating a specific population of highly specialized memory T cells, which are crucial for long-lasting immunity, remains a challenge. Finally, even successful regeneration may not fully restore the diverse repertoire of T cell specificities that are present in a healthy individual.
- Age-related decline in thymic function
- Inability to fully compensate for severe T cell loss
- Challenges in regenerating specific T cell subsets
What is the future of T cell regeneration research?
The field of T cell regeneration research is dynamic and rapidly evolving. Further research focuses on understanding the complex molecular mechanisms regulating T cell development and differentiation to identify potential therapeutic targets. Advanced technologies, such as CRISPR-Cas9 gene editing, hold promise for enhancing T cell regeneration or even generating customized T cells with specific desired functionalities. Clinical trials are exploring new methods for thymic rejuvenation and improving the effectiveness of adoptive T cell therapies. The long-term goal is to develop effective strategies to restore or enhance T cell function in various disease settings, including immunodeficiencies, cancer, and infections.
- Investigation of molecular mechanisms of T cell development
- Development of novel therapeutic strategies using gene editing technologies
- Clinical translation of promising research findings to improve patient care
How long does it take for T cells to recover?
How Long Does It Take for T Cells to Recover?
The time it takes for T cells to recover depends significantly on several factors, including the severity and nature of the immune challenge, the individual's overall health, and the presence of any underlying conditions. There isn't a single definitive answer. Recovery can range from a few weeks for mild infections to several months or even longer for more severe illnesses or immunosuppressive treatments. The recovery process isn't simply a matter of cell count but also encompasses the functional capacity of the T cells – their ability to recognize and respond to antigens. This functional recovery can be slower than the replenishment of cell numbers.
Factors Influencing T Cell Recovery Time
Numerous factors play a crucial role in determining how long it takes for T cells to recover. These factors can significantly influence the speed and completeness of recovery. A healthy immune system generally recovers faster than a compromised one. Age also plays a significant role, with older individuals often exhibiting slower recovery times. The type and severity of the illness or treatment also directly impact the duration of recovery. The presence of any co-morbidities or underlying medical conditions can further complicate and slow the recovery process.
- Severity of the Immune Challenge: A mild viral infection will lead to much faster recovery than a severe case of sepsis or a bone marrow transplant.
- Individual's Overall Health: Nutritional status, the presence of chronic diseases, and age can all influence recovery time.
- Type of Immunosuppression: The intensity and duration of immunosuppressive therapies, such as chemotherapy or corticosteroids, drastically affect T cell recovery.
Measuring T Cell Recovery
Assessing T cell recovery involves several methods. Direct measurement of T cell counts in the blood (through blood tests) is a common approach, but this only offers a partial picture. More sophisticated techniques assess T cell function through assays that measure their ability to proliferate, produce cytokines, or kill target cells. These functional assessments provide a more comprehensive understanding of immune recovery than simple cell counts alone. The results of these tests are essential for guiding clinical management decisions.
- Complete Blood Count (CBC): Provides a basic assessment of overall immune cell numbers.
- Flow Cytometry: A more detailed analysis of different T cell subsets and their activation status.
- Functional Assays: Measure the ability of T cells to perform their immune functions.
T Cell Subset Recovery
Not all T cell subsets recover at the same rate. Naive T cells, which haven't encountered an antigen, might take longer to replenish than memory T cells, which have previously encountered a pathogen. This difference reflects the different roles these subsets play in the immune response. The differential recovery rates of various T cell subsets can have important implications for overall immune competence. Monitoring these specific subsets is crucial for a comprehensive understanding of recovery.
- Naive T cells: These cells are responsible for initiating primary immune responses and take longer to recover.
- Memory T cells: These cells provide immunological memory and recover more quickly.
- Regulatory T cells (Tregs): These cells suppress immune responses and their recovery is important for preventing autoimmunity.
Nutritional Support and T Cell Recovery
Proper nutrition plays a crucial role in supporting T cell recovery. A balanced diet rich in protein, vitamins, and minerals is essential for optimal immune function. Specific nutrients, such as zinc, vitamin D, and antioxidants, are particularly important for supporting T cell proliferation and function. Deficiencies in essential nutrients can impair the body's ability to regenerate and repair its immune system, lengthening the recovery period. Supplementation may be considered under specific circumstances and should be discussed with a healthcare professional.
- Protein: Essential for building and repairing cells.
- Vitamins: Various vitamins are crucial for immune cell function.
- Minerals: Minerals such as zinc and selenium are important for immune cell development.
Impact of Medical Interventions on T Cell Recovery
Various medical interventions can influence T cell recovery times. Immunosuppressive drugs, chemotherapy, and radiation therapy all suppress the immune system, significantly delaying T cell recovery. Conversely, some therapies, such as cytokine therapy, aim to boost T cell activity and accelerate recovery. The choice of intervention and its impact on T cell recovery must be carefully considered by healthcare professionals on a case-by-case basis.
- Immunosuppressive drugs: Used to prevent organ rejection or treat autoimmune diseases, but they suppress T cell function.
- Chemotherapy and radiation therapy: These treatments can severely damage the bone marrow, reducing T cell production.
- Cytokine therapy: This therapy can stimulate T cell proliferation and activity, potentially speeding up recovery.
What stimulates the growth of T cells?
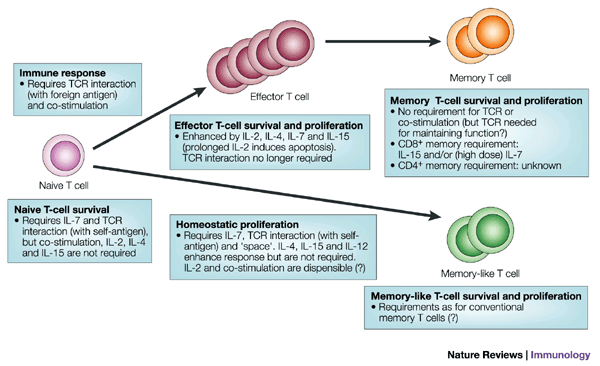
T cell growth and development are complex processes driven by a variety of factors, primarily involving interactions between the T cell and its surrounding environment. These factors act at different stages of T cell development and activation, influencing proliferation, differentiation, and ultimately, the generation of a functional T cell repertoire capable of mounting an effective immune response. Signaling through the T cell receptor (TCR) is arguably the most crucial stimulus, triggered by the binding of a specific antigen presented on the surface of an antigen-presenting cell (APC). This initiates a cascade of intracellular signaling pathways that are essential for T cell activation, survival, and proliferation.
Cytokines
Cytokines, soluble signaling molecules, play a pivotal role in regulating T cell growth. Different cytokines promote various stages of T cell development and differentiation. Interleukin-2 (IL-2), for example, is a potent T cell growth factor crucial for the expansion of activated T cells. Other cytokines like IL-4, IL-7, and IL-15 also contribute to T cell development and maintenance. The specific mix of cytokines present in the microenvironment dictates the type of T cell response that develops (e.g., Th1, Th2, Th17, Treg).
- IL-2: Drives clonal expansion of activated T cells.
- IL-7: Essential for the development and survival of naive T cells.
- IL-15: Supports the survival and homeostasis of memory T cells and natural killer (NK) cells.
Antigen Presentation
The presentation of antigens by antigen-presenting cells (APCs) is fundamental to T cell activation and subsequent growth. APCs, such as dendritic cells, macrophages, and B cells, process and present antigenic peptides bound to major histocompatibility complex (MHC) molecules on their surface. The interaction between the TCR on the T cell and the MHC-peptide complex on the APC is the initiating event that triggers T cell activation, leading to proliferation and differentiation. The nature of the antigen, the MHC molecule, and the co-stimulatory signals provided by the APC all influence the subsequent T cell response.
- MHC Class I: Presents intracellular antigens to CD8+ cytotoxic T cells.
- MHC Class II: Presents extracellular antigens to CD4+ helper T cells.
- Co-stimulatory molecules (e.g., B7): Provide essential signals for T cell activation.
Co-stimulatory Signals
Beyond TCR engagement, co-stimulatory signals are crucial for effective T cell activation and growth. These signals are usually provided by APCs through the interaction of co-stimulatory molecules on the APC surface (e.g., B7) with their receptors on the T cell surface (e.g., CD28). The absence of co-stimulation can lead to T cell anergy (unresponsiveness) or apoptosis. Co-stimulation ensures that T cells are activated only when encountering a genuine threat, preventing inappropriate immune responses.
- CD28: A major co-stimulatory receptor on T cells.
- B7 (CD80/CD86): Co-stimulatory ligands expressed by APCs.
- CTLA-4: A negative regulator of T cell activation, counteracting CD28.
Growth Factors and Hormones
Beyond cytokines, several other factors influence T cell growth. Growth factors, such as insulin-like growth factor-1 (IGF-1) and transforming growth factor-beta (TGF-β), can modulate T cell proliferation and differentiation. Hormones, such as glucocorticoids, can influence T cell development and function, often exerting immunosuppressive effects. The interplay between these factors and the immune system is intricate and crucial for maintaining immune homeostasis.
- IGF-1: Promotes T cell proliferation.
- TGF-β: Can suppress T cell activation and promote Treg cell differentiation.
- Glucocorticoids: Have immunosuppressive effects on T cells.
The Microenvironment
The tissue microenvironment surrounding the T cell significantly influences its growth and function. The presence of other immune cells, extracellular matrix components, and chemokines all contribute to the overall milieu in which T cells develop and respond to stimuli. For example, the presence of certain chemokines can recruit T cells to specific tissues, while the composition of the extracellular matrix can influence T cell migration and activation. The interplay of signals within this complex environment is essential for a properly regulated immune response.
- Chemokines: Direct T cell migration to sites of inflammation.
- Extracellular matrix: Influences T cell migration and adhesion.
- Other immune cells: Provide additional signals and interactions that modulate T cell responses.
What are T cells and why would they need rebuilding?
T cells are a crucial component of the adaptive immune system. They are a type of white blood cell that plays a central role in cell-mediated immunity, recognizing and eliminating infected or cancerous cells. There are various types of T cells, each with specific functions, including helper T cells (Th cells) that orchestrate the immune response, cytotoxic T cells (Tc cells) that directly kill infected cells, and regulatory T cells (Treg cells) that suppress the immune response and prevent autoimmunity. Rebuilding, or more accurately, replenishing or restoring, T cells becomes necessary in several scenarios. These include situations where the T cell population is severely depleted, such as in advanced HIV infection, certain cancers, or following intense immunosuppressive therapy. Furthermore, in cases of immunodeficiency, the body's ability to produce functional T cells might be compromised. Autoimmune diseases, paradoxically, can also benefit from targeted T cell manipulation, where specific populations of harmful T cells are depleted or reprogrammed. In essence, the need to "rebuild" T cells arises when their numbers are insufficient, their function is impaired, or their activity is misdirected, leading to compromised immunity or harmful autoimmune reactions. Current research explores various strategies to achieve this, including immune checkpoint inhibitors, adoptive cell therapies (ACT), and gene therapies.
How can T cells be "rebuilt" or replenished?
The concept of "rebuilding" T cells encompasses several complex approaches. It's not a simple process of "rebuilding" like fixing a machine, but rather manipulating and restoring the body's ability to generate and maintain a healthy T cell population. One major strategy is adoptive cell therapy (ACT). In ACT, T cells are harvested from a patient, modified in the laboratory to enhance their anti-tumor activity or to target specific antigens, and then re-infused back into the patient. This is particularly useful in cancer treatment, where modified T cells, such as CAR T cells (Chimeric Antigen Receptor T cells), can effectively eliminate cancer cells. Another approach involves manipulating the bone marrow, the primary site of T cell development. Stem cell transplantation can restore the bone marrow's ability to produce healthy T cells, although this is usually a last-resort option for severe immunodeficiencies. Further, research focuses on stimulating the body's own mechanisms for T cell production and maturation. This involves strategies that modulate the immune microenvironment to foster T cell growth and differentiation. Immunomodulatory drugs can also indirectly support T cell restoration by influencing the balance of different immune cells and reducing inflammation. Finally, gene therapy holds considerable promise, allowing for the correction of genetic defects that impair T cell development or function. These approaches are not mutually exclusive; often, combined strategies are employed to achieve optimal results.
What are the challenges in rebuilding T cells?
Rebuilding or restoring T cell function presents significant challenges. One major obstacle is the complexity of the immune system. T cell development and function are intricately regulated by a complex network of interactions between various cell types and signaling molecules. Manipulating this system without causing unintended consequences is a significant undertaking. Furthermore, off-target effects are a considerable concern in many approaches, such as ACT. Modified T cells might inadvertently attack healthy cells, leading to serious side effects. Toxicity is another hurdle, particularly with certain types of immunotherapy or gene therapy. The procedures themselves can be demanding and carry risks, particularly for patients with compromised immune systems. The cost of these advanced therapies is also substantial, limiting accessibility for many patients. Finally, the effectiveness of T cell rebuilding varies widely depending on the underlying condition, the patient's overall health, and the specific therapeutic approach used. Research continues to address these challenges, aiming to improve the safety, efficacy, and affordability of T cell-based therapies.
What is the future of T cell rebuilding technology?
The field of T cell manipulation and restoration is rapidly evolving. Future advancements are expected to focus on several key areas. Improved targeting is crucial to minimize off-target effects and enhance the specificity of T cell therapies. This could involve advanced genetic engineering techniques and novel approaches to identify and target cancer-specific antigens or other disease-related markers. Enhanced persistence of modified T cells in the body is another major goal. Researchers are exploring ways to make modified T cells more durable and less susceptible to elimination by the immune system. Development of safer and more effective methods of T cell expansion and modification will also be critical, with a focus on reducing the risk of toxicity and improving the manufacturing processes. Furthermore, personalized approaches based on the individual patient's genetic profile and immune status are likely to become increasingly important. The integration of artificial intelligence (AI) and machine learning in the design and optimization of T cell-based therapies holds immense potential. Finally, exploring alternative sources of T cells, such as induced pluripotent stem cells (iPSCs), could provide a more readily available source of T cells for therapeutic use. These advances are expected to lead to more effective and safer treatments for a wide range of diseases, including cancer, infectious diseases, and autoimmune disorders.
Deja una respuesta